Summary:
Electrospray Ionization (ESI) is a key technique in LC-MS, enabling the gentle ionization of thermally labile and polar compounds, particularly useful for analyzing biomolecules like small molecules & proteins. Innovations such as the pneumatically assisted nebulizer have stabilized the process and optimized it for liquid chromatography. ESI mechanisms include the Ion Evaporation Model (IEM) for small molecules, the Charged Residue Model (CRM) for globular proteins, and the Chain Ejection Model (CEM) for unfolded proteins. Derivatization techniques, like adding charged groups or enhancing surface activity, improve ESI signal strength, especially for small analytes. Advanced ion sources, such as the TurboV, feature adjustable heating gases, and ceramic elements to ensure uniform heating and efficient ion generation. Ongoing advancements aim to enhance reliability and sensitivity in mass spectrometric analyses.
Electrospray ionization (ESI) is currently the most widely utilized interface in liquid chromatography-mass spectrometry (LC-MS). As a gentle ionization technique, ESI enables the study of thermally labile and polar compounds. It is particularly valuable for analyzing biomolecules like proteins, as it facilitates the generation of multiply charged molecular ions.
The feasibility of using ESI for producing biomolecular ions was first demonstrated by Fenn et al., building upon earlier work by Dole et al. in the 1960s, who constructed the first electrospray-based mass spectrometer. Fenn et al. later employed an ESI tandem quadrupole detector to detect molecular beams, identifying deficiencies in Dole’s ion source design. They improved the setup by adjusting the distance between the ion source needle and the end plate, thereby reducing the required needle voltage. This led to the development of the Fenn-Whitehouse design, which utilized a glass capillary to convey ions from the atmospheric environment into the first vacuum chamber. The choice of capillary diameter varied according to specific experimental needs.
In 1987, Bruins et al. introduced a pneumatically assisted nebulizer to enhance the electrospray interface. This innovation helped stabilize the electrospray process by maintaining a flow rate compatible with liquid chromatography, typically around 0.2 mL/min, ideal for tandem ESI-MS applications. By increasing the distance between the spray nozzle and the counter electrode, occurrences of corona discharge were minimized. Additionally, this adjustment reduced the dependency of the spraying process on the nozzle position, potentially leading to increased sensitivity when the nozzle was positioned off-axis.
Since these developments, the structural design of ESI sources has undergone numerous refinements, leading to a better understanding of their operational principles.
1.1 Mechanism of Electrospray Ionization
Electrospray ionization (ESI) is a technique that utilizes an electric field to generate charged droplets, which are subsequently desolvated to produce gas-phase ions for analysis by mass spectrometry. The process involves three primary stages: the formation of charged droplets, the contraction of these droplets, and the ultimate generation of gas-phase ions.

In ESI-MS, the sample solution is delivered through a capillary at a low flow rate, typically ranging from 0.1 to 10 μL/min. A high voltage, usually between 2 and 5 kV, is applied to the capillary, which can be either positive or negative depending on the nature of the analyte. This voltage creates an electric field gradient necessary for the separation of charges on the surface of the liquid. In the presence of this electric field, the liquid forms a “Taylor cone” at the tip of the capillary (Figure 1).
While the exact mechanism for the formation of the Taylor cone remains somewhat unclear, it has been observed that the shape of the cone depends on the capillary voltage and is influenced by the pulsation of the fluid within the capillary (Figure 2). When the solution at the tip of the Taylor cone reaches the Rayleigh limit—the point where the Coulomb repulsion of the surface charge balances the surface tension of the solution—a highly charged droplet is formed at the cone’s apex.
As the solvent evaporates and the droplet shrinks, the repulsive forces between the charges increase. Once the Rayleigh limit is exceeded, the droplet undergoes a Coulomb explosion, ejecting excess charge and forming smaller charged droplets. These newly generated droplets continue to undergo subsequent rounds of evaporation and explosion, leading to the production of even smaller droplets and eventually gas-phase ions.
The mechanism of how gas-phase ions are generated from very small droplets is not yet fully understood, but three main models have been proposed:
(1)The Ion Evaporation Model (IEM), first proposed by Iribarne and Thomson, suggests that ions evaporate from the surface of the charged droplets.
(2)The Charged Residue Model (CRM), proposed by Dole et al., posits that ions are ejected from the droplets due to the repulsion between charged residues.
(3)A newer theory, the Chain Ejection Model (CEM), proposed by Konermann et al., offers an alternative explanation for the ionization process.

(a) IEM: ejection of small ions from the surface of charged microdroplets;
(b) CRM: release of globular proteins into the gas phase;
(c) CEM: ejection of unfolded proteins;
It is generally accepted that the transfer of small molecules to the gas phase follows the Ion Evaporation Model (IEM). These analytes are charged through a protonation process facilitated by organic acids present in the solution. When the electric field applied to a charged droplet is sufficiently strong, evaporation occurs under the influence of the surface ionic potential, and a single charged, solvated analyte enters the gas phase.
The Charged Residue Model (CRM) is applicable to larger, globular substances such as naturally folded proteins. In neutral aqueous solutions, most proteins appear as compact spheres, with charges and polar groups located on the exterior to maximize hydrophilicity. The nonpolar portion forms a hydrophobic core that does not interact with the solvent. According to Konermann et al., this spherical protein with a hydrophilic exterior and hydrophobic interior follows the CRM. The theory posits that after successive Coulomb fission, a droplet containing a single analyte is formed. When the solvent layer evaporates, the charge of the droplet is transferred to the analyte molecules, forming gas-phase ions.
Molecular dynamics modeling suggests that unfolded proteins follow a distinct mechanism during electrospray ionization, known as the Chain Ejection Model (CEM). Conditions such as acidic mobile phases trigger the protein structure in solution to unfold, resulting in a highly disordered conformation. Nonpolar groups that were previously isolated within the interior become exposed to the solvent, causing the protein to change from a compact hydrophilic state to a loosely hydrophobic one. The hydrophobic nature of the highly unfolded protein makes it unsuitable to remain within the droplet, leading it to migrate to the droplet surface. The ends of the chain are then expelled into the gas phase, followed by the sequential and progressive expulsion of the remainder of the protein until it separates from the droplet. Although CEM shares some similarities with the IEM, it is fundamentally different from the CRM mechanism. CEM is suitable for disordered, partially hydrophobic, chain-like polymers capable of accommodating excess charge carriers.
Understanding the properties of the analyte is crucial for the successful application of ESI and for determining the ESI response. The following section will discuss the analyte properties that affect the ESI response and summarize methods to enhance the ESI signal strength of weakly responsive substances.
1.2 Ionization Modes
Enke et al. systematically categorized the ionization modes of electrospray ionization (ESI) into the following four categories:
Charge Separation Ionization: In ESI, charge separation is primarily used for ionizing inorganic substances, biomolecules with acidic or basic groups, compounds containing amines, phosphorus, or carbonyl groups, and proteins with various amino acid residues that can readily form ions through protonation.
Adduct Formation: Adding salts at appropriate concentrations can facilitate the formation of adducts, enabling the ionization of polar compounds lacking acid-base groups. This process occurs before charge separation and is used to generate positive or negative ions.
Gas-Phase Reaction Ionization: Charged analytes exiting a charged droplet and entering the gas phase can be further charged through interactions with other gas-phase ions, such as solvent molecular ions, via proton transfer reactions. This process favors analytes with higher proton affinity energies.
Ionization by Electrochemical Redox Reactions: Electrochemical reactions can convert uncharged analytes into ionic forms, which can then be ionized. However, some electrochemical reactions may have adverse effects, such as overcharging the analyte, oxidizing the contacting metal, and altering the electrochemical reaction.
1.3 Factors Affecting the ESI Response
The properties of the analyte directly influence the ionization efficiency in the ESI source, which in turn affects the signal response. Background noise can also interfere with the response. Iribarne et al. first proposed that nonpolar analytes with high surface affinity tend to exhibit higher ESI responses. Kebarle et al. and Apffel et al. suggested that the ESI response is related to the rate of evaporation from the surface of the ESI droplet based on the theory of ion evaporation. Enke concluded that, regardless of the ion evaporation rate, only analytes capable of forming an excess charge on the surface of an ESI droplet are responsive to ESI. Therefore, the higher the likelihood that an analyte will participate in the surface excess charge, the higher its ESI response will be.
1.3.1 Effect of analyte properties on ESI response
During the electrospray ionization process, the surface of the parent droplet tends to form a cone shape and subsequently produces daughter droplets. It is therefore assumed that analytes with surface activity are more likely to acquire charge. Tang et al. demonstrated this theory using fluorescent substances as samples, showing that the smaller sub-droplets at the periphery of the ESI spray were enriched with surfactants, whereas non-surface-active substances were uniformly distributed throughout the ESI spray.
In addition to surface activity, several property-related parameters can predict the suitability of an analyte for ESI. For instance, the ESI response is linearly related to the nonpolar surface area of a peptide. Enke et al. demonstrated that peptides with more nonpolar (hydrophobic) side chains exhibited the highest mass spectral response. This finding was corroborated by Zhou et al., who found that phenylalanine, which contains nonpolar amino acids, showed a significant enhancement in response compared to serine, which has a polar group.
Furthermore, the ESI response is also related to the Gibbs free energy of transfer from nonpolar to polar solutions. Analytes with higher Gibbs free energies (i.e., more nonpolar analytes) tend to have higher responses. For simple, singly-charged analytes, there is a relationship between HPLC retention time and ESI response. Nonpolar analytes with higher responses are retained more strongly by HPLC in reverse-phase mode. For large, multiply charged peptides and proteins, the relationship between ESI response and HPLC retention time remains an area of ongoing research. However, there is already evidence suggesting that peptides with a greater number of nonpolar groups have a stronger ESI response compared to those with more polar groups.
1.3.2 Enhancing ESI Signal Through Derivatization
For electrospray ionization (ESI) sources, derivatization can be used to improve chromatographic separation, ionization efficiency, and detection signals. Specifically, the response of the ESI source to an analyte can be enhanced by making the analyte more susceptible to charging or by increasing its surface activity. Most derivatization reactions involve introducing charged groups onto neutral molecules to facilitate ion formation through the creation of sodium adducts or enhanced protonation. Another approach is the introduction of electrochemically reactive groups within the molecular structure, where ionization is achieved through electrochemical redox reactions of the derivatives. Nonpolar groups can also be added to unresponsive analytes to enhance the surface activity of the droplets, thereby boosting the ESI signal.
Currently, the enhancement of the ESI signal for small analytes through derivatization has garnered increasing attention. Large molecules, such as peptides, can be conjugated to organic small analytes. Since peptides are multiply charged, contain many nonpolar amino acids, and are highly responsive to ESI, they can significantly enhance the response of small organic molecules. Additionally, the formation of adducts increases the molecular weight of the analytes, which can help avoid interference from background small molecules and thus improve the signal-to-noise ratio
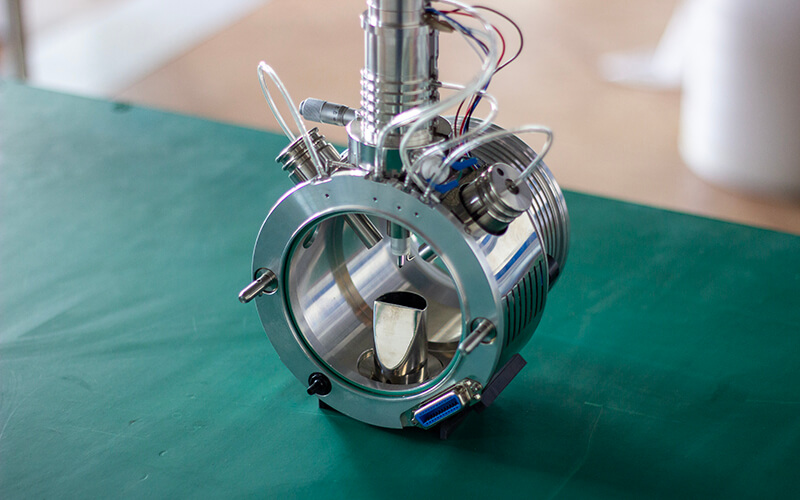
2 The TurboV Ion Source and Its Mechanism
The TurboV ion source from Sciex is widely used in the industry, and we offer ion sources with similar interfaces.
The TurboV ion source features two orthogonal auxiliary heating gases, with a flow rate range of 0-17 L/min, which is adjustable. The heating head employs ceramic heating elements and ceramic beads to achieve a more uniform heating effect of the gas. The newly added gas flow intersects with the initial gas flow, promoting turbulent mixing and enhancing the evaporation of liquid droplets. This approach minimizes the circulation of gas and liquid within the ion source, improving the efficiency of ion generation.
Turbulence contributes to the ionization process by enhancing the instability of the electric field, thereby facilitating ion formation. Specifically, turbulence introduces spatial and temporal heterogeneity to the electric field. This inhomogeneity can lead to localized areas of significantly enhanced electric field strength. When the electric field strength exceeds a certain threshold, neutral atoms or molecules can absorb sufficient energy to break their electron bonds, leading to ionization.
Moreover, turbulence enhances ionization by promoting collisions and mixing between particles. Within turbulent regions, the random motion of particles is intensified, increasing the likelihood of collisions and energy exchange. Such collisions can excite neutral particles to higher energy levels, making them more prone to ionization.


Fig. 3 Schematic of AB Sciex Ion Source structure
3 Our Ion Source Compatibility and Features
We can provide an exact match for the ion source, including the manufacturer’s gas connections (GAS1, GAS2, and BATH GAS), as well as compatible installation connectors.
Electrically, the 24 pins on the ion source are calibrated to ensure proper connectivity and functionality.
Features:
- Complete supply of ESI and APCI probe accessories.
- Adjustable probe positioning.
- ESI voltage up to 5500V.
- Calibrated temperature rise curve for the heating rod, enabling stable temperature regulation up to 750°C.
- Auxiliary heating gas outlets are filled with ceramic beads to ensure uniform temperature distribution.
Our ion sources offer consistent replacement across all aspects, including identification, heating, gas paths, power supply, and functionality.
When your ion source experiences performance degradation due to long-term use or suffers damage from discharge caused by incorrect operation or environmental anomalies, our products can be quickly replaced to ensure that your laboratory operations proceed smoothly


Pingback: Unit – III [M. Pharm – I – Semester (Pharmaceutics)] – “Being beyond the best”